Nature Photonics publication on a new imaging technique to illuminate cells
Sep 17, 2025
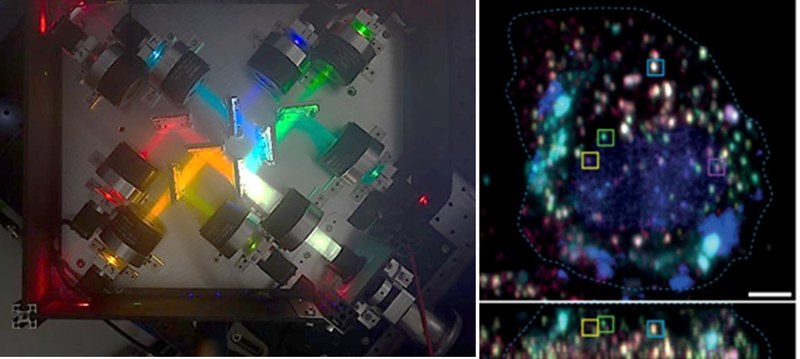
Dr. Guillem-Marti, along with researchers from the Baker lab (USA) and the Manton lab (UK), has just published a new study in Nature Photonics introducing a new methodology for simultaneously acquiring eight spectral channels from any camera-based fluorescence microscope.
Fluorescence microscopy is a powerful tool for studying the dynamics of life, providing images of cellsand tissues at the molecular level. However, the broad spectra of typical fluorophores used for biological imaging limit the number of colors to three or four. Accordingly, only two or three cellular components can typically be studied in the same sample.
The paper describes a breakthrough method that let watch many different components in live cells at once without losing clarity. Using an 8-channel camera module and a novel algorithm, this system can track up to seven fluorescent tags in three dimensions and over time, even when signals are dim.
Noteworthy, this approach can be easily incorporated into any spinning disk confocal microscope. To see a real application in action, custom-designed minibinders, each labeled with bright organic dyes, were used to follow how cell-surface receptors are sorted after they enter the cell. In living cells, it was possible to observe which receptors end where during endosome splitting, allowing to track them inside cells. This was achieved without the necessity of genome-editing the endogenous receptors.
This advance opens up the possibility for studying complex interactions inside cells, like how signals are routed or how subcellular organelles coordinate, with unprecedented resolution in both space and time.
For more information and detail, read the full paper, available in OPEN ACCESS: A. Kumar, K.E. McNally, Y. Zhang, A. Haslett-Saunders, X. Wang, J. Guillem-Marti, D. Lee, B. Huang, S. Stallinga, R.R. Kay, D. Baker, E. Derivery, J.D. Manton. Multispectral live-cell imaging without uncompromised spatiotemporal resolution. Nat. Photonics 2025. https://doi.org/10.1038/s41566-025-01745-7.
Share: